Autoimmune diseases (e.g., Systemic lupus erythematosus, Malignant rheumatoid arthritis, Guillain-Barré syndrome, Chronic inflammatory demyelinating polyneuropathy, Multiple sclerosis)
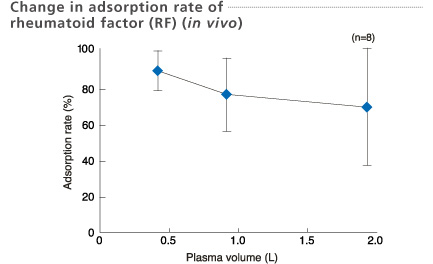
Plasma samples were collected at the inlet and outlet of the column.
Data: Kamifukuoka Sogo hospital, Seisuikan Hospital
Ohashi et al. Jpn J Apheresis 13(2) : 201-202, 1994
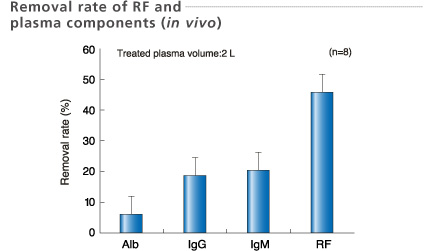
Blood samples for removal rate were collected before and after treatment.
Data: Kamifukuoka Sogo hospital, Seisuikan Hospital
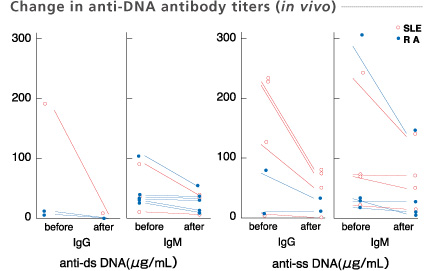
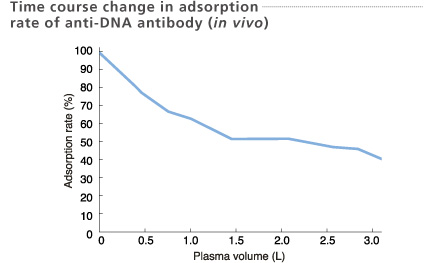
Plasma samples were collected at the inlet and outlet of the column.
Okudaira et al. Therapeutic Plasmapheresis Ⅳ, 149-152, 1984
In this case, a 48-year-old female with a 5-year-history of RA (stage 3, class 3), experienced relief from joint pain and the disappearance of rheumatoid nodules. The clinical improvements accompanied with the shown laboratory findings, which were maintained for over 3 months.
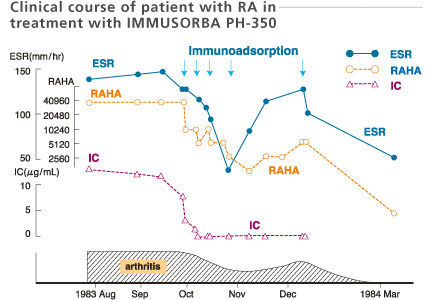
RAHA : Rheumatoid arthritis hemagglutination assay
IC : Immune complexes
Kobayashi et al. Therapeutic Plasmapheresis Ⅳ, 153-157, 1984
| Adsorbent | Material | Phenylalanine immobilized polyvinylalcohol gel |
|---|---|---|
| Volume | 350 mL | |
| Priming Volume | 300 mL | |
| Container | Material | Polypropylene |
| Dimension | 211mm[L] x 62mm[D] | |
| Weight | 650 g | |
| Sterilization | High pressure steam | |
| Filter | Material | Polyethylene (coated with ethylene-vinylalcohol copolymer) |
|---|---|---|
| Area | 0.07 m2 | |
| Container | Material | Poly (vinyl chloride) |
| Dimension | 165mm[L] x 22mm[D] | |
| Priming Volume | 30 mL | |
| Sterilization | Ethylene oxide | |
For patients undergoing treatment with angiotensin-converting enzyme (ACE) inhibitor, there is a possibility that treatment with the IMMUSORBA PH-350 will lead to a drop in blood pressure. Simultaneous treatment with ACE inhibitor and the IMMUSORBA PH-350 must be avoided.
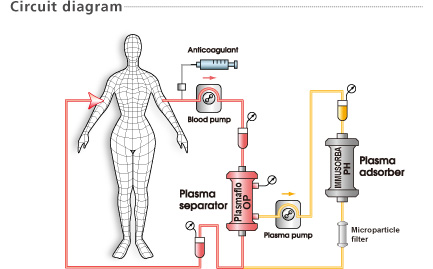
The IMMUSORBA PH-350 is intended for the treatment of plasma. Never run whole blood through the IMMUSORBA PH-350. Thrombocytes cannot pass through the IMMUSORBA PH-350 and may cause blockage.
Do not use IMMUSORBA PH-350 with plasma containing a large amount of thrombocytes.